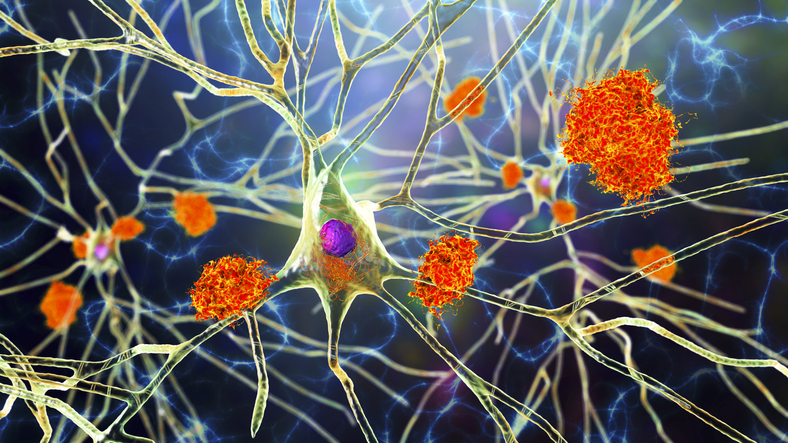
Scientists have linked the progression of Alzheimer’s to the buildup of specific proteins in the brain. One of the best-known of these Alzheimer’s biomarker proteins is called beta-amyloid, which builds up in the brain in a thick plaque that gets in the way of neurons, eventually causing them to die. There are a now a number of drugs — both on the market and in the drug development pipeline — that target beta-amyloid as a way of stopping or slowing Alzheimer’s disease.
However, other problematic proteins might be involved in Alzheimer’s and other dementias, too, and scientists are sounding alarms that these lesser-known proteins deserve a closer look. In 2006, researchers found a new protein deposit implicated in dementia: TAR DNA binding protein (TDP-43), which tends to show up in clumps in the brains of people with frontotemporal dementia and amyotrophic lateral sclerosis.
TDP-43 and Alzheimer’s
Gradually, researchers learned that TDP-43 also aggregates in the brains of about half of all people with Alzheimer’s. Compared to healthy older individuals, there are lower levels of TDP-43 in the blood of some people with frontotemporal dementia. In Alzheimer’s, these levels are even lower.
TDP-43 isn’t always the bad guy: In a healthy brain, its role is to help copy the genetic instructions for making proteins. But if the TDP-43 gets moving to the wrong location in the cell, it starts clumping up and could lead to eventual neurodegeneration. It can no longer do the job of supporting communication between healthy brain cells.
Scientists haven’t yet learned what makes these proteins go haywire, but they consider them essential contributors to the disease.
Targeting TDP-43 to Treat Dementia
Drug developers are looking to TDP-43 as a possible target for drugs designed to help slow or stop dementia. Drugmaker Annovis Bio is currently developing one such drug, buntanetap, that prevents TDP-43 buildup as an approach to stopping disease progression.
Buntanetap comes in capsule form. It works by halting the formation of several of these known, problematic protein plaques and deposits, including amyloid, tau, and TDP-43.
It will soon commence Phase 3 trials for treating the mild and moderate stages of Alzheimer’s, Parkinson’s, and other neurodegenerative diseases.
Other researchers are developing gene therapies to compensate for losing healthy TDP-43. While TDP-43 is still in brain cells, it isn’t doing its job since it’s clumped up, further accelerating cell death in the brain. This type of gene therapy can help support the survival of neuronal cells in mouse models of neurodegenerative disease. Similar kinds of treatments might eventually make their way to human trials.
A new wave of Alzheimer’s treatments for the disease — including the recently FDA-approved Leqembi and Aduhelm — are betting that lowering the levels of beta-amyloid plaques will slow the progression of the disease. But many researchers also believe that the future of treating Alzheimer’s and dementia involves more than one drug — targeting multiple pathways… and multiple proteins. Buntanetap and other drugs that target TDP-43 could be important pieces to solving the bigger Alzheimer’s puzzle.