Getting an accurate Alzheimer’s diagnosis can take years. That’s because, unlike conditions like diabetes or high blood pressure, Alzheimer’s doesn’t have a single, straightforward test.
Instead, the process begins by ruling out other possible causes of memory loss or confusion — such as side effects from medications or vitamin and hormone deficiencies. Only after those are excluded do doctors look for physical signs of Alzheimer’s in the brain: the buildup of two proteins — beta-amyloid and tau — that are linked to the disease.
In recent years, the U.S. Food and Drug administration has authorized three main tools to detect these biomarkers: cerebrospinal fluid tests (performed with a spinal tap), brain scans (specifically, PET scans), and, most recently, quick, simple blood tests that can screen for these Alzheimer’s biomarkers. Here’s how each one works.
CSF tests: The original biomarker test
The first method developed to detect Alzheimer’s biomarkers involves collecting cerebrospinal fluid (CSF), which surrounds the brain and spinal cord. Doctors use a procedure called a spinal tap to collect the fluid. While it can be uncomfortable, many patients say it’s not especially painful.
Once the sample is collected, labs measure the levels of beta-amyloid and tau proteins — especially the ratios of pTau-181 to Aβ42, tTau to Aβ42, or Aβ42 to Aβ40. Abnormal ratios can indicate Alzheimer’s. These tests are currently covered by Medicare.
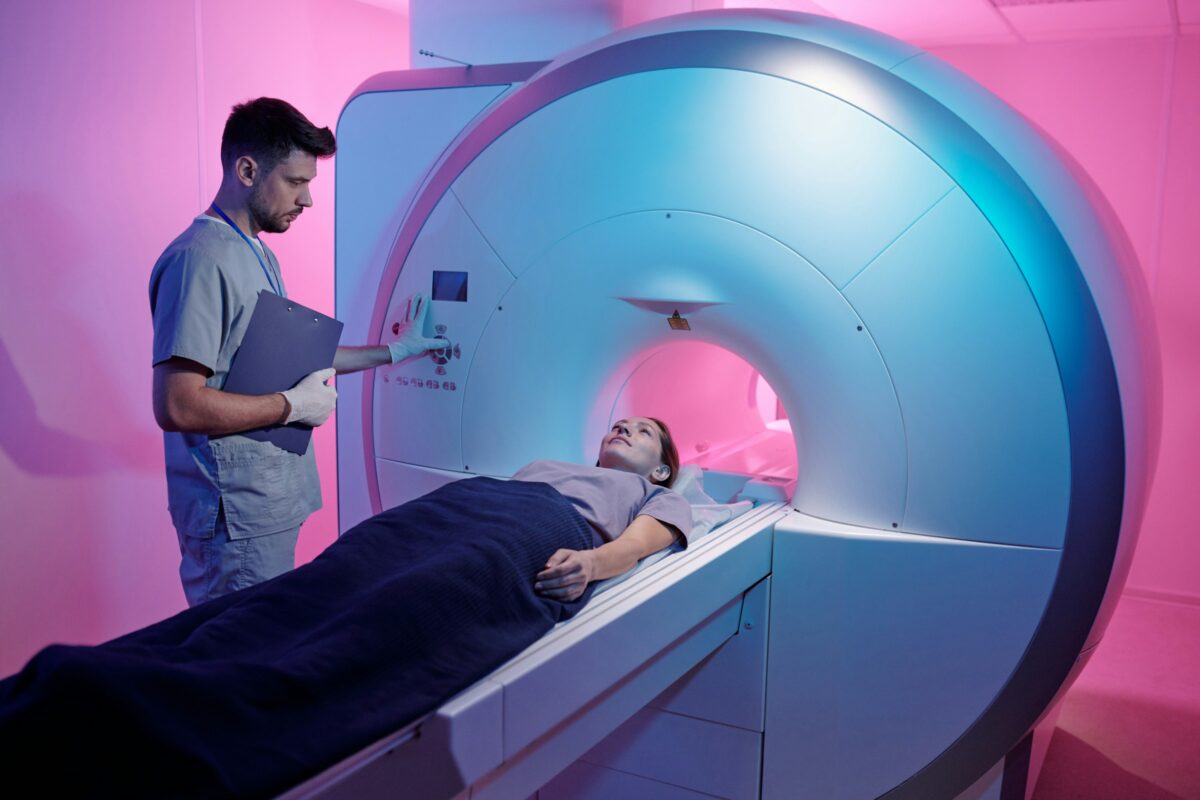
PET scans: Brain imaging for amyloid and tau
The next major advance came in the form of PET (positron emission tomography) scans. In this test, a small amount of a radioactive tracer is injected into the body. It travels to the brain and attaches to beta-amyloid plaques. A scan then shows where these plaques have built up.
Several different FDA-approved tracers are available for beta-amyloid PET scans, and these scans are also covered by Medicare.
Scientists have also developed PET tracers that detect tau. While tau PET scans are promising — especially for tracking disease progression or determining who might benefit from treatment — they’re not yet widely used. They’re expensive and less accessible than other methods.
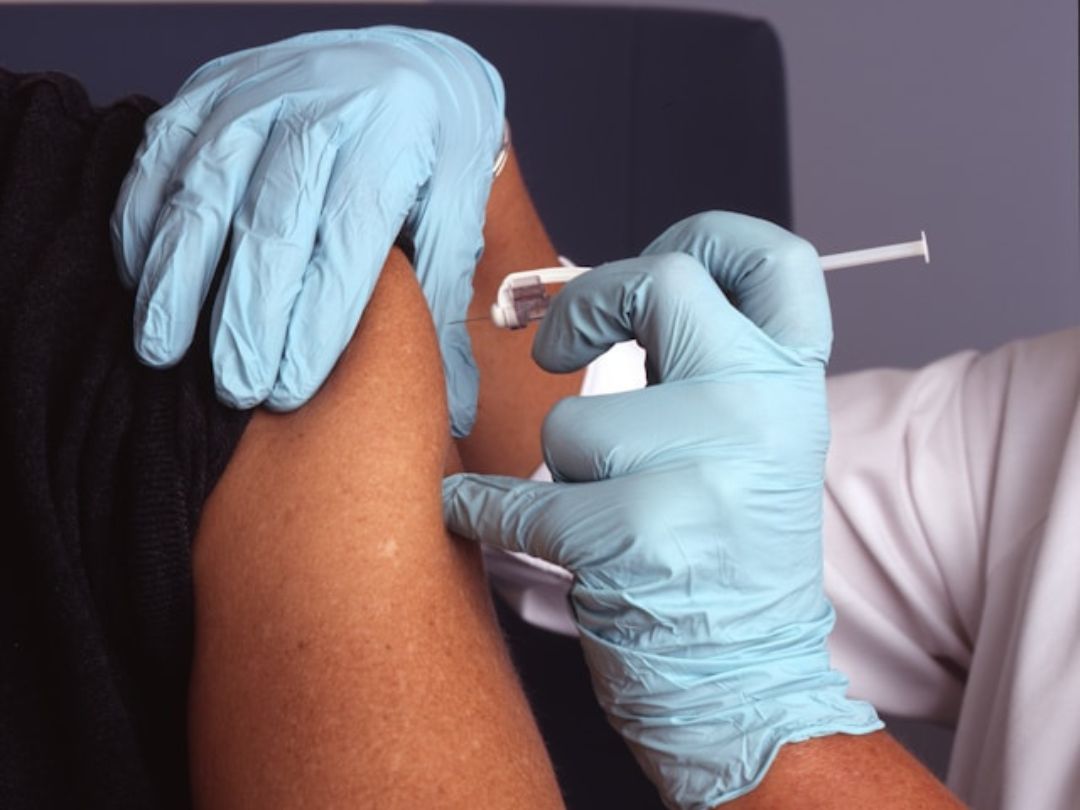
Blood tests: The newest, most accessible option
The newest and most convenient option is a blood test. These tests measure proteins related to Alzheimer’s, like pTau-217, and can help indicate whether someone should move on to a CSF test or PET scan for confirmation. Some are accurate enough to provide confirmation on their own.
That said, blood tests are not meant for screening healthy people — they’re used when there are already signs of cognitive decline.
There are more than a dozen different Alzheimer’s biomarker blood tests currently on the market. One of them — developed by the company Fujirebio — is approved by the FDA. Fujirebio’s test measures the ratio of pTau-217 to Aβ42 in people over age 50 and is designed for use by neurologists evaluating memory or cognitive concerns. Medicare is expected to begin covering it in January.
Why an early diagnosis matters
As less invasive and more accessible tests like blood work become more common, they could help people get diagnosed earlier — when it matters most.That’s because new Alzheimer’s drugs like Leqembi and Kisunla are only approved for people in the early stages of the disease. Getting a timely diagnosis gives patients and their families more options: to consider treatment, to plan for the future, or to participate in clinical trials. Explore trials enrolling now at K2.